Cold Working and Hot working | Residual Stresses | Recovery and Re-Crystallization
Cold Working and Hot working | Residual Stresses | Recovery and Re-Crystallization
Cold Working
What is Cold Working?
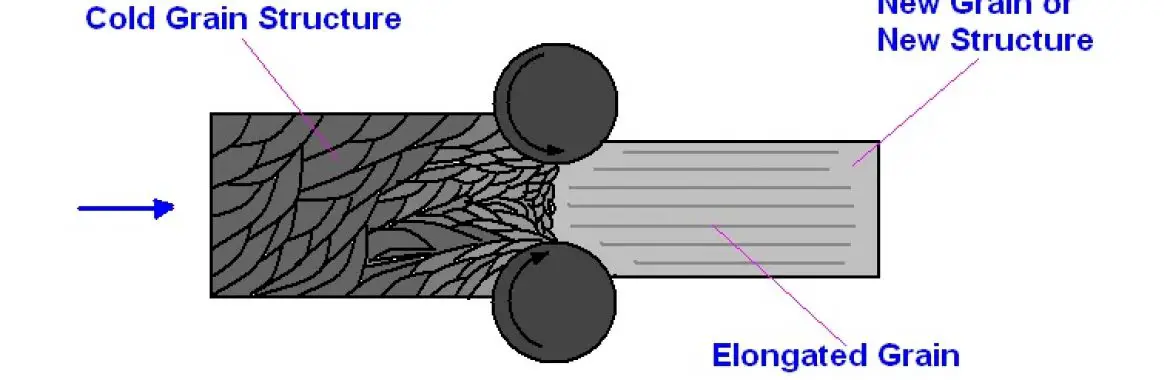
When a metal is subjected to a mechanical process like drawing, cold rolling, bending, etc., it undergoes plastic deformation below the re-crystallization temperature. The process is termed as cold working. Most of the cold working processes are performed at room temperature. Since the process is below the re-crystallization temperature, there is no re-crystallization of grains.
Residual Stresses
What is Residential Stresses?
Strength and hardness increase with a corresponding decrease in ductility while the metal is deformed; it gives rise to severe stresses inside the metal, known as residual stresses. These stresses are due to the piling up of dislocations near barriers on the slip plans like grain boundaries.
These residual stresses increase the hardness and corrosion resistance of the metal required. These stresses can be removed by a suitable heat treatment below the re-crystallization temperature metals lose their stored energy during the process of recovery, re-crystallization, and regain original structure and properties.
The pressure (mechanical energy) required in cold working is more than that required in hot working, e.g., cold working for tungsten is 12000C as against 8000C hot working for steel. The metal is not deformed permanently until the stress exceeds the elastic limit of the material.
It gives a better surface furnish and closer dimensional tolerances than by hot working. The metal will fracture if the cold is excessive. Therefore, it is done in stages with intermediate annealing operations to soften the metal and restore ductility.
💥🎁 Christmas & Year-End Deals On Amazon !
Don't miss out on the best discounts and top-rated products available right now!
🛒 Shop Now and Save Big Today!*As an Amazon Associate, I earn from qualifying purchases.
Since most of the cold working is carried out at an ambient temperature, the use of soaking pit and furnaces is avoided. There is no handling of hot materials. Hence it is a faster process. There is no loss of metal due to oxidation. However, the energy required to deform the metal is more.
Hot working
What is Hot Working ?
When a metal is subjected to a hot working process, it undergoes plastic deformation above the re-crystallization temperature. The process is called Hot Working. During this process, the metal is in a plastic state, and it readily takes the desired shape. Because of higher temperature scaling, oxidation of the metal surface takes place.
Forging, hot drawing, hot cupping, hot extruding are some of the hot working processes.
Advantage of Hot Working
- Hot working helps in elimination of porosity and blowholes.
- Refinement of coarse grains.
- Improvement in physical properties.
- Increase in strength and better homogeneity.
- Better resistance to impact loading.
- Improvement in ductility.
During hot working, most metals are in plastic condition; hence pressure required informing is less. Therefore, less power is needed, compared to cold working. However, hot working has got certain limitations. Surface finish and dimensional tolerances are poorer than those for cold working.
💥🎁 Christmas & Year-End Deals On Amazon !
Don't miss out on the best discounts and top-rated products available right now!
🛒 Shop Now and Save Big Today!*As an Amazon Associate, I earn from qualifying purchases.
Also, the cost of equipment and handling is high because the material is hot. Structure and other properties are not uniform throughout the cross-section of metals.
Recovery/ Stress Reliving) and Re-crystallization.
Recovery (Stress Reliving)
What is Recovery?
The process by which the strain-free state of the cold-worked metal is achieved by heating without significant changes in its microstructure is known as recovery or stress relieving. It is relatively a low-temperature process, below the melting point, and is essential for releasing internal stresses in forgings, welded and fabricated equipment, boiler tubes, without affecting the strength achieved during cold working.
Electrical conductivity and ductility of materials increase rapidly towards the annealing value during recovery. The internal stress is also reduced significantly. The strength and hardness properties controlled by dislocations are not affected at recovery temperature, but it increases the metal’s ductility. Dislocations that pile up during cold working rearrange themselves and get reduced during recovery. This is known as polygonization.
Re-Crystallization
Because of residual stresses, a work-hardened material tends to regain its symmetrical lattice structure. During heat-treatment, new grains are formed, and re-crystallization takes place.
The increased heating time and cold work were done earlier, lowers the temperature required for re-crystallization. The formation of new grains during this process reduces the internal forces, decreases mechanical strength and hardness, and recoups the ability to withstand plastic deformation.
Comparison Recovery and Re-Crystallization.
What is Re-crystallization temperature?
The temperature at which about 50% of the cold-worked metal re-crystallizes in one hour is known as re-crystallization temperature. Re-crystallization temperature for some of the common materials is given below:
💥🎁 Christmas & Year-End Deals On Amazon !
Don't miss out on the best discounts and top-rated products available right now!
🛒 Shop Now and Save Big Today!*As an Amazon Associate, I earn from qualifying purchases.
Aluminum – 1500C Magnesium – 1500C
Copper – 2000C Iron – 4500C
Nickel – 6200C Silver – 2000C
Steel – 8000C Tungsten – 12000C
Comparison between recovery and recrystallization Table
💥🎁 Christmas & Year-End Deals On Amazon !
Don't miss out on the best discounts and top-rated products available right now!
🛒 Shop Now and Save Big Today!*As an Amazon Associate, I earn from qualifying purchases.
Recovery |
Re-crystallization |
|
| 1. | It involves lower temperature. | It involves higher temperature. |
| 2. | It restores strain free state without any change in micro-structure of the cold worked metal. | Cold worked structure is replaced by a new strain free grain structure |
| 3. | There is no change in grain size. | There is slight increase in grain size. |
| 4. | Internal stresses are released without decreasing the strength achieved by cold working. | Internal stress is completely removed. |
| 5. | Electrical conductivity and ductility are increased rapidly towards the annealing value during recovery. | There is a slow improvement in electrical conductivity and sharp increase in ductility. |
| 6. | Strength and hardness are not affected | It results in a decrease in hardness and strength. |
| 7. | Dislocation of the same slip planes align themselves into walls to form small angle sub-grain boundaries and arrange themselves during recovery. | The elimination of sub-grain boundaries is a basic part of a re-crystallization process. |
| 8. | Rearrange of dislocations and lowering of lattice energy takes place due to above. | The driving force for re-crystallization comes from the stored energy of cold work. |
Grain Growth
When the new strain-free grains obtained after re-crystallization of worked metal are heated at a temperature higher than that required to cause re-crystallization, a progressive increase in grain size is achieved. This is known as grain growth. It is temperature-dependent. In a polycrystalline material, the yield stress (σy) increases with a decrease in grain size; as per the following equation
σy = σi + kd-½
where σi = Yield stress for a crystal having no grain boundaries.
D = Grain diameter, K = Hall Petch constant
💥🎁 Christmas & Year-End Deals On Amazon !
Don't miss out on the best discounts and top-rated products available right now!
🛒 Shop Now and Save Big Today!*As an Amazon Associate, I earn from qualifying purchases.
When the temperature is increased above the re-crystallization, these grains grow in size. The grain growth takes place due to the combination of individual grains, thereby reducing their boundary area ( grain boundaries), and grains become stable (See figure above)
Grain growth depends upon several factors such as annealing temperature and time, degree of previous cold work, the effect of insoluble mixtures to the metal, addition of certain alloying elements, rate of heating and cooling.
Grain size is affected considerably by the fine dispersion of second phase particles. They restrict grain boundary movement.
There is a slight decrease in hardness and strength values during grain growth. There is a further increase in electrical conductivity and ductility of the metal. Surface appearance is also governed by grain size.
Grain Boundaries
What is a Grain Boundary?
A grain boundary can be defined as the interface between two grains, or crystallites, in a polycrystalline material like metal. Grain boundaries are 2D defects in the crystal structures that occurs where two such crystallites meet and the same crystal structure and chemical composition exists on each side but the orientation differs. This boundaries tend to decrease the thermal and electrical conductivity of the material.
💥🎁 Christmas & Year-End Deals On Amazon !
Don't miss out on the best discounts and top-rated products available right now!
🛒 Shop Now and Save Big Today!*As an Amazon Associate, I earn from qualifying purchases.